Applications
Our software can be used for many applications in the laboratory; some examples are shown below. As the software is configurable you can organise your own laboratory data management system based on your specific requirements, while still maintaining compliance to any regulatory standards.
A wide cross-section of templates for data collection are supplied with the software. These can be used out-of-the-box or modified by you. The data templates can then be used to build applications, from equipment metrology to studies. Terrington Data Management provides full support post-implementation to help you build and protect the applications you require.
Applications include:
Labnotes
LABNOTES was created specifically for use in bioanalytical contract research laboratories. A set of forms and assets, supplied with software, have been developed for targeted use within this sector. These cover studies (small and large molecule), analytical standards, stock solutions, reagents, chemicals, control matrix, equipment and devices. All the applications shown below are also included in your LABNOTES package.
In addition, core functionality can be applied allowing LABNOTES to be compliant with the guidance set-out in (FDA) 21 CFR Part 11 (providing SOPs are followed). LABNOTES is in use in GxP environments and customers using LABNOTES have been successfully audited by sponsors and regulatory bodies.
Study reports with integration to Watson LIMs and LABNOTES can be generated using our affiliated product, OPAL.
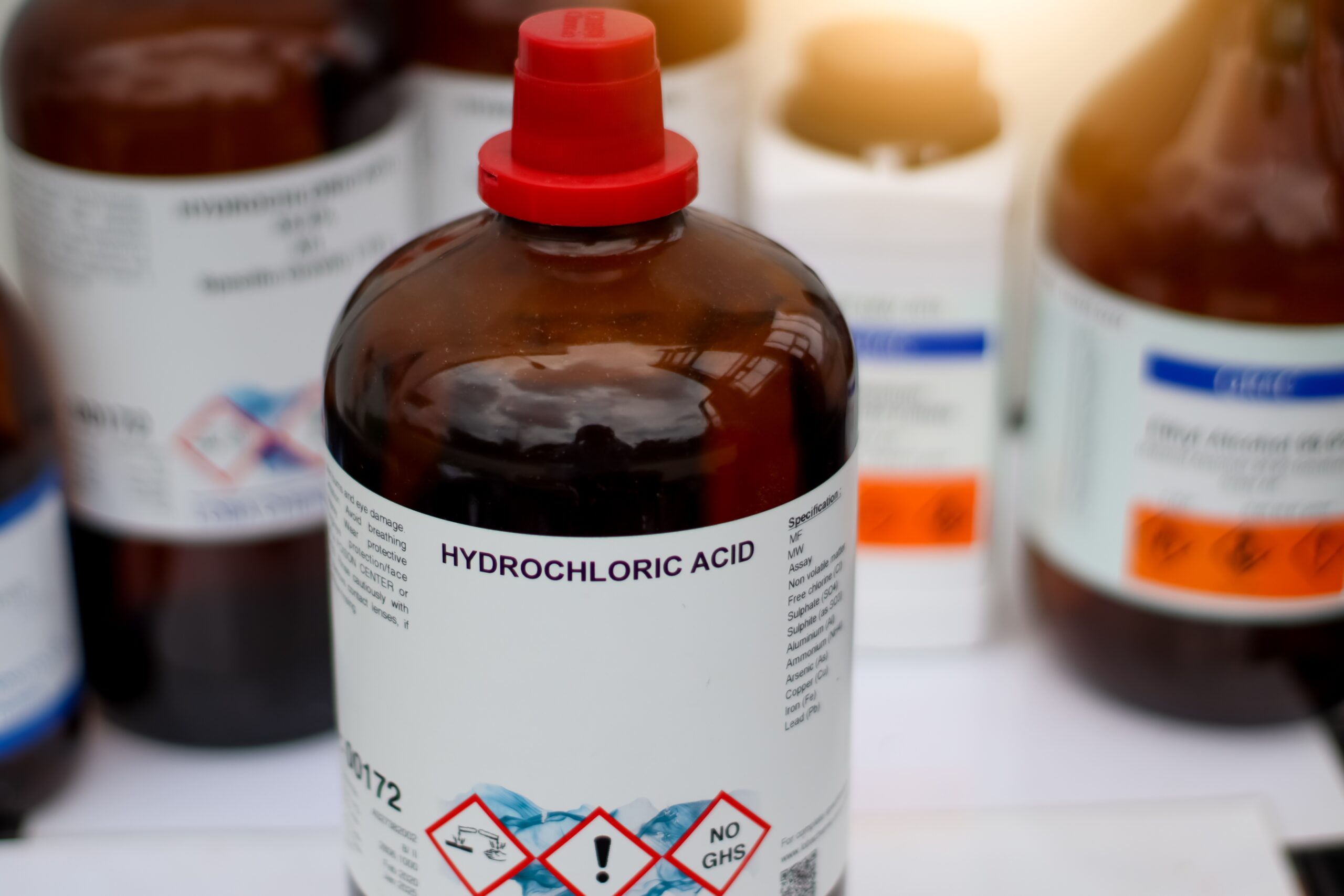
Chemical and reagent management
Our software can be used for managing all your chemicals and reagents, providing an end-to-end solutions from log-in inventory, to usage and disposal.
Safety (COSHH and MSDS) information data sheets can be attached to any inventory and stored in the document library. Methods for the preparation of any reagent can be controlled and log any chemical used. Each reagent preparation is automated with the operator only required to enter minimum data. Labels and barcodes can be created for each chemical and/or reagent.
Document storage
A document library is supplied with the software. You can upload any of your reference documents to the library. All documents in the library are stored in the software database. A document (any file type) in the library can be referenced against a set of operations as a “Doc Ref” or attached to a data template. Operators can view the referenced documents if they have permission to do so.
Within the library you can create a file structure, upload additional references, review attachment location(s) and search for any document in the library. The document library also has user permissions so access can be controlled by the system administrator.
Instrument inventory
An instrument inventory application can be used to control, monitor, record calibrations and maintenance for any equipment used in your laboratory. Any “out of specification” instrument can be flagged-up and cannot be used without recoding a reason for use or until it is re-calibrated/inspected. “Critical Fields” can also be deployed which if changed render the instrument “unapproved for use” and requiring re-approval by management.
Any external maintenance records can be attached to the instrument and stored in the document library. Inventory reports can be generated to show the entire history of an instrument from purchase to retirement.
